MMI Co-Founder, John Hipp, PhD. to be Guest Editor for Special Issue of Bioengineering! Submit by May 30!
Learn MorePress Release!: Driving Excellence in Cardiovascular Trials: MMI and Healthcare Inroads Deepen Collaboration
Read HereTherapeutic Area Expertise
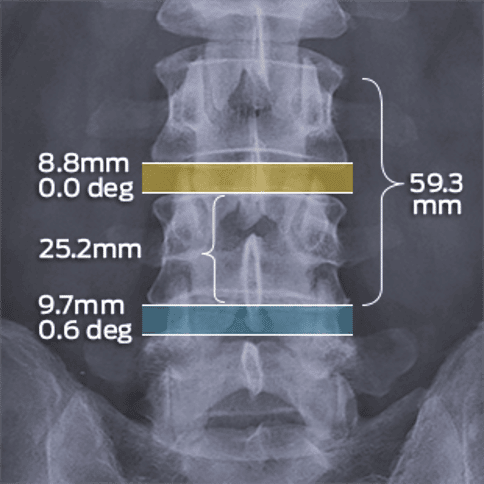
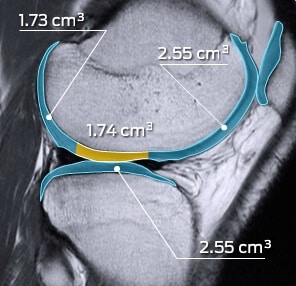
MMI’s therapeutic area expertise includes spine, orthopedics, cardiovascular, neurology, ENT, GI, urology, oncology studies, and more!
OUR EXPERTISE
Leaders in Clinical Imaging Design and Interpretation
Since 2000, MMI has partnered on hundreds of trials of new medical devices, drugs, and biologics across a range of therapeutic areas. Our experience spans all study types and phases, ranging from preclinical and early-phase feasibility testing to post-market surveillance.
MMI supports oncology clinical trials via the MMI-Micron Collaboration. Our partnership delivers robust imaging expertise across 20+ read criteria, including RECIST 1.1 and cancer-specific response evaluations. Our PET imaging specialists offer advanced capabilities in dosimetry, novel PET tracer quantification, and theranostic applications, helping sponsors unlock deeper insights from complex studies. With a global footprint and streamlined workflows, we ensure the rapid turnaround times critical to the success of oncology trials.
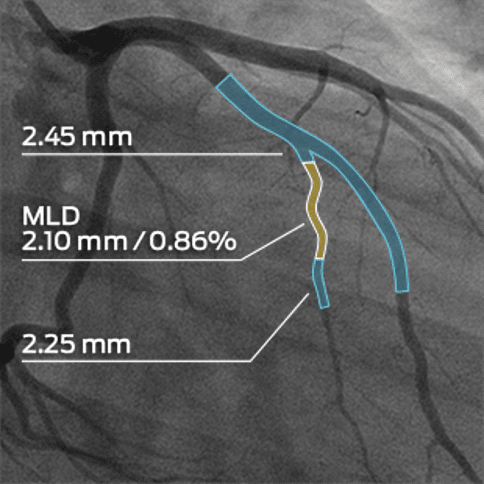
MMI’s experience in the cardiology, endovascular, and peripheral vascular spaces allows us to address the specific imaging needs of any trial. We work with luminary experts in the field and support all major cardiovascular imaging modalities, including echocardiography, angiography, CT, and MRI, in a single core lab. Our cardiovascular experience includes peripheral vascular disease, atherectomy, thrombectomy, arterio-venous fistula, aneurysms, stents, drug delivery catheters, and more.
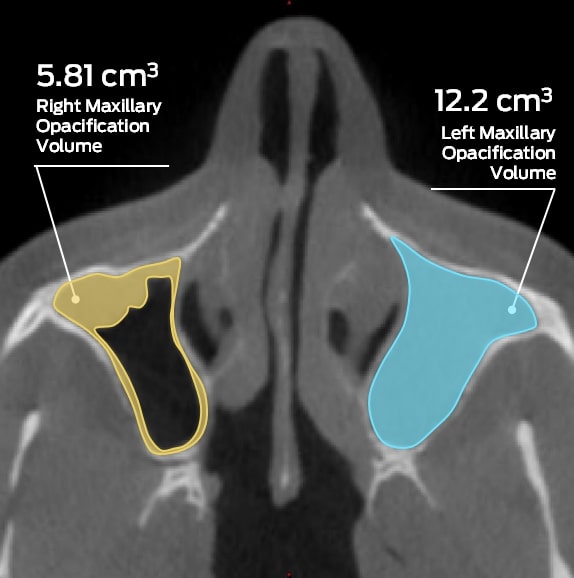
As one of the only imaging core labs supporting ear, nose, and throat clinical trials, MMI has validated and customized analysis methodologies in support of multiple, regulated clinical trials. We have the ability to objectively and quantitatively characterize pathologies such as sinus opacification, which may be missed using more traditional, qualitative grading systems. Our ENT experience includes chronic sinusitis, allergic rhinitis, nasal polyps, nasal irrigation, drug-eluting stents, and more.
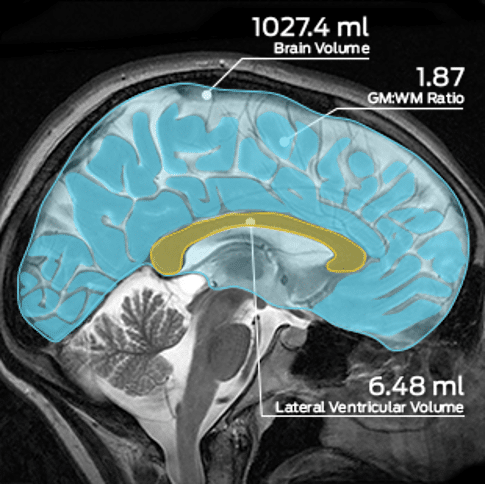
MMI’s technical staff have over 50+ years of combined experience in neuroimaging. We support all major diagnostic neuroimaging modalities in a single core lab, and have validated tools for advanced image analysis and volumetric quantification. Our neurology experience includes aneurysm, acute ischemic stroke, traumatic brain injury, embolic protection, brain tumor therapies, and more.
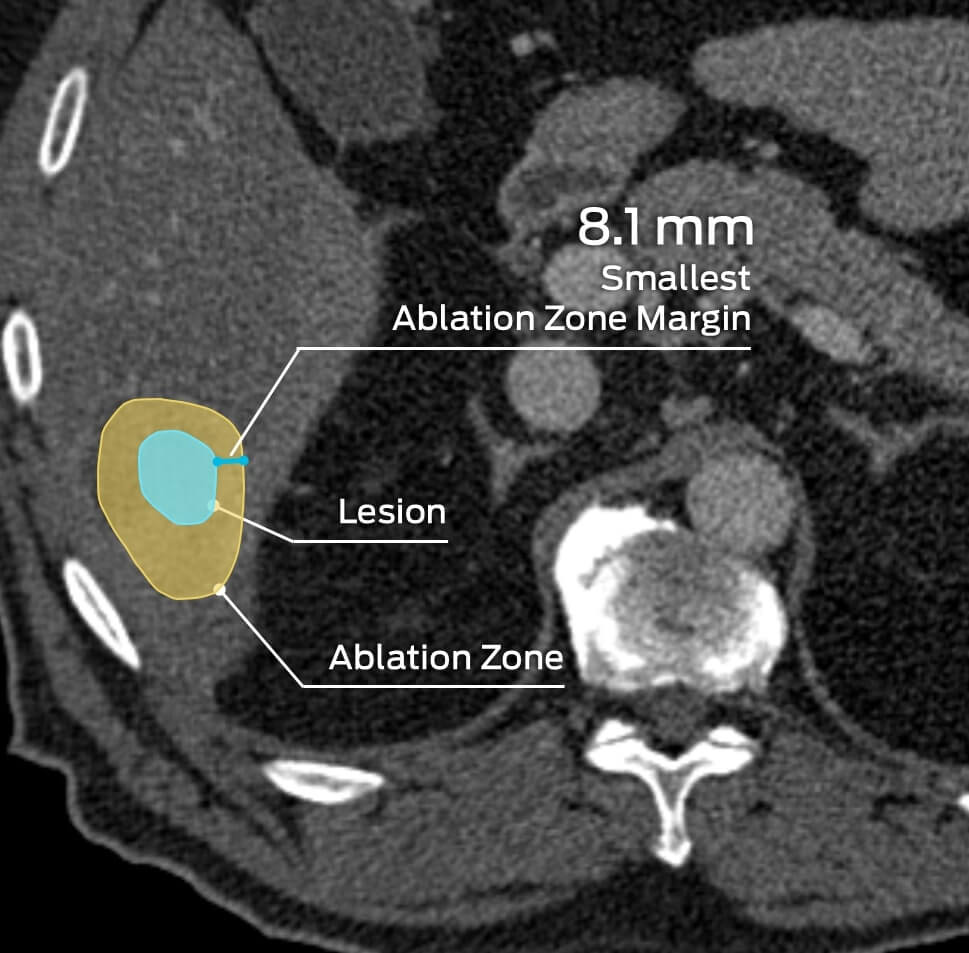
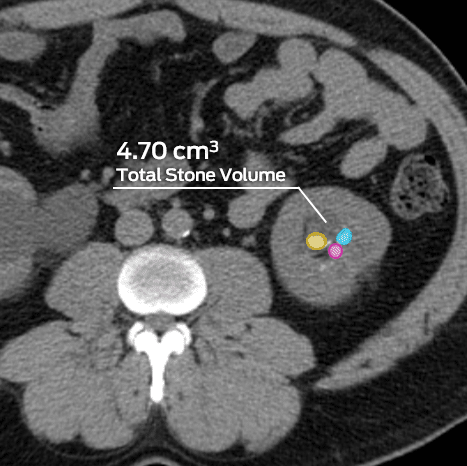
MMI has supported numerous clinical trials in the gastrointestinal and urology spaces, including indications involving the liver, kidneys, and other abdominal soft tissue and organ systems. MMI’s experience includes soft tissue and organ ablation therapies, kidney/urinary stone treatments, aneurysms, emboli, nodule identification, surgical repair assessments, and more.
AI Research
MMI has extensive experience in running artificial intelligence (AI) clinical trials and research studies, including R&D, pilots, ground truthing and standalone trials. MMI has managed multiple AI trials in the orthopedic space, including releasing its own FDA cleared Software as a Medical Device (SaMD), SpineCAMPTM. Services range from study & protocol design, data analysis and interpretation.
Frequently Asked Questions
What kind of experts will be interpreting my images?
All of our expert reviewers are practicing, licensed, and board-certified physicians with both clinical and research interests. Depending on the study needs, these experts may be radiologists, surgeons, or other types of clinicians with subspecialty training in a particular therapeutic area (e.g. musculoskeletal radiologist or interventional cardiologist). MMI will select the appropriate reviewers to participate in your study based on your trial design and pre-defined reviewer selection criteria.
Can you support trials outside of the listed therapeutic areas?
Potentially! MMI has the infrastructure and staff to support a variety of trial designs and imaging modalities. In addition, we have a strong network of key opinion leaders in different therapeutic areas to provide clinical and scientific expertise. If your trial is in another therapeutic area, contact us to learn more about our ability to support your trial! We value scientific integrity and honesty, and will always provide you with a candid response.
Let MMI provide insights into your clinical study imaging.
Have questions? We’ll connect you immediately to one of our scientific managers and imaging experts. Your time is precious, and we want to make the most out of it.